In the course of thrombosis in the pelvic vein, scarring and obstruction often occur. Since the venous valves are involved, they can lose their function. This leads to blood congestion in the legs.
Typical consequences of pelvic vein obstruction or occlusion are varicose veins, painful swelling of the legs, calf cramps and poorly healing venous ulcers.
Modern, non-invasive diagnostic procedures can precisely locate these obstruction and occlusions.
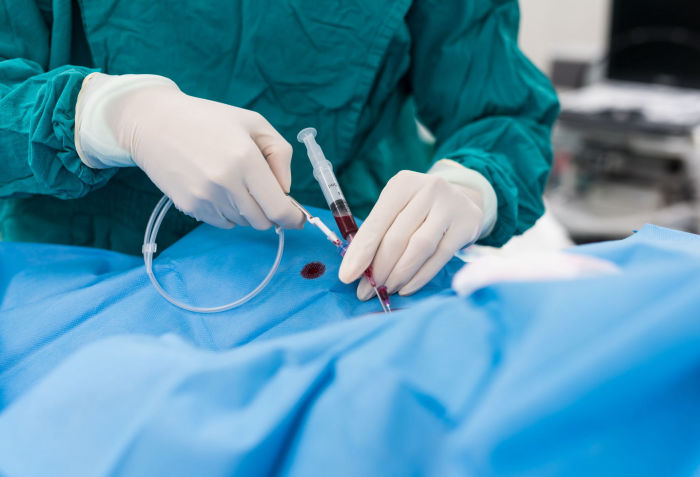
If an acute or chronically occluded or severely obstructed pelvic vein has been diagnosed as the cause, it can be reopened by a minimally invasive catheter procedure.
In the pelvic vein area, a venous stent is then advanced under radiological control up to the narrow point and released there. In the case of extensive occlusions, it is sometimes necessary to implant several or one long stent.
Due to the reopening of the pelvic vein the blood flow in the direction of the heart is improved. This reduces the backflow in the legs and thus the pressure on the tissue. After this treatment, open ulcers have the chance to heal and massive swelling of the legs can be reduced. The reopening of occluded pelvic veins is a complex procedure that requires a great deal of experience and a venous stent that fulfils special requirements.
These minimally invasive procedures are performed in special centers.